Excuse me waiter, there is Mercury in my fish!
Is Mercury(Hg) bad or evil? If so where does it come from and how do I avoid it. And how did it get into my fish! A narrative journey with highlights from my clinic, lab and current research.
A gentleman is about to eat his meal at a popular restaurant when he exclaims, excuse me waiter, there is Mercury in my fish!
I’m not surprised sir. Anything else I can help you with, says the waiter.
Well I am surprised and horrified and I’m sure you know Mercury is very toxic and I want to know - how did it get into my fish.
Yes it is very toxic Sir. You may have noticed that all the lighting in our restaurant is now LED, while before it was from compact fluorescent bulbs that often released Mercury into our land and water when disposed of.
The energy that keeps these bulbs bright actually comes from the coal fired power station a few hundred kilometres away and emits mercury into the atmosphere that then deposits into the oceans. And that lovely gold wedding ring you are wearing Sir may contain gold that came from an artisan gold mine not far down the coast that we know contaminates the river with Mercury which then flows in to the ocean. However, no need to worry Sir as we test our fish every now and then to make sure it conforms to the levels of Mercury allowed by our government regulatory authorities.
That’s ridiculous and preposterous. And just can’t be.
The man, obviously attracted to eating the delicious looking fish in front of him, is at the same time thinking - what I really want to know is - what amount of Mercury in my body is harmful to me!
Exactly. That’s what this article is about. An investigation into how much Mercury is harmful for a human being like myself who occasionally desires to eat fish and recently tested positive for traces of mercury. How are we exposed and how did it get to this. It’s an honest journey of discovery accompanied with little premonition of the outcome.
By the way, while a fictional story above, everything the waiter said is factual.
Additionally, in the next few articles we will be putting Lead (Pb), Cadmium (Cd) and Arsenic (As) under the microscope as they exist in Soil, Dust and Water. When is enough, enough. Let’s get started.
The main character in this story is Mercury
SYNONYMS are: Hg(2+) | Hg2+ | Mercuric ion | Mercury ion | Mercury(2+) | Mercury(2+) ion | Mercury(II) | Mercury(II) cation | Mercury(II) ion | Metallic mercury | Quicksilver
The atomic symbol for Mercury is Hg, which comes from Greek name, Hydrargyrum, meaning “liquid silver”. The atomic number is 80 (mercury has 80 protons in the nucleus) and the atomic weight or mass is approximately 201 (rounded up from 200.59, which means mercury has 121 neutrons). Note that while protons and neutrons are approximately equal in mass, the disparity in the maths is due to a phenomenon known as binding energy. Keeps the atom intact to some degree. Mass and energy are interchangeable.
We will need the atomic mass number (200.59 ) for Mercury later when we make sense of Australian urinary laboratory test results for mercury presented in nmol/L.
Mercury occurs in deposits throughout the earth, often as cinnabar (mercuric sulphide) which, when crushed forms the red pigment, vermilion.
Mercury bio-accumulates in human brain, the food chain and the environment and is highly toxic. Pregnant-to-be woman, the elderly and children, and especially developing foetuses and infants, are particularly vulnerable to mercury. Here we demonstrate that mercury exposure occurs through multiple possible routes and that individuals can reduce exposure significantly via the simple knowledge presented here.
To aid in the gathering of evidence based clinical research, our resident Master of Medical Librarianship, has conducted a literature review with the following parameters - Search topic: Clinical guidelines and health based cut-off points for non-occupational Mercury (Hg) levels detected in urine, blood and hair in adults and children - particularly young children and pregnant/lactating mothers.
It is also worth declaring that in preparation for this writing and my own experience over the last few decades, it has imerged that things are not black and white. Even in “evidence based research” you and I will come across contradictory and conflicting results that make it difficult to come to a definite conclusion. This is in part because we don’t actual know as much as we are often lead to believe. A simple deep dive into ANY scientific topic will demonstrate within a few short hours or days that you have hit the wall, the limit to our knowledge and understanding in that particular field or subject.
It is essential to keep an open curious mind, flushed clean daily of biases, beliefs and fears. This helps tremendously with the ability to ask the right questions. You don’t have to be a science nerd like myself to benefit from this daily mind purging. Off course regular walking, sleeping, good eating and meditating assists in mind clutter disposal.
It is encouraged to comment and ask questions. Others will benefit from this. We will update this article regularly with new knowledge taken from your comments and learnt from your questions.
Areas Covered
PART 1: What are the main sources of exposure to Mercury
PART 2: What are the health consequences from being exposed to Mercury.
PART 3: How do I know I have been exposed to Mercury - details of Hair, Urine and Blood Tests. How do I get it out of my body. How do I know how much Mercury is going to cause me problems and if not now, will it bite me later. This is getting personal now.
Canthigaster valentini (fish species) by Jenny Huang
Main Sources of Mercury Exposure for Humans
The primary route of mercury exposure for the general population is through consumption of contaminated fish and seafood. The other sources outlined below can also be significant for exposed individuals. The most devastating tragedies related to Mercury (Hg) toxicity in recent history include Minamata Bay and Niagata, Japan in the 1950s, and Iraq in the 1970s.
Dietary Intake:
Seafood and Fish: Mercury, particularly in the form of methyl mercury (MeHg) (We explain later what this is), accumulates in marine and freshwater fish, especially in top predators such as tuna, swordfish, and sharks. This is one of the most significant sources of exposure for humans.
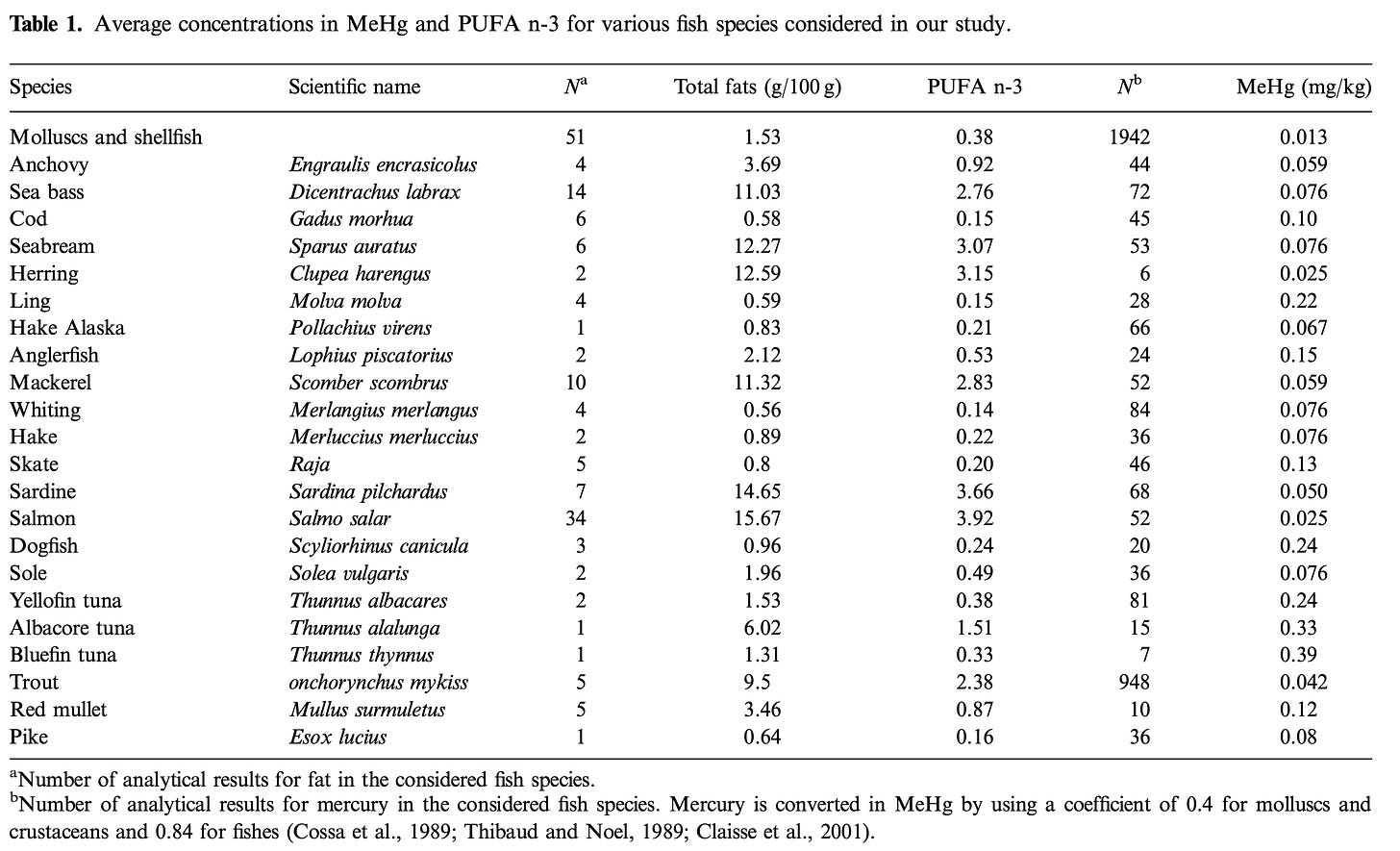
Table below shows amounts of Methyl Mercury (MeHg) expressed as mg/kg in selected fish species. The last column shows the amount of Mercury as MeHg. Many tables like this have been published in the literature since. Some like this one also associate the amount of omega 3 fatty acids available in the fish so as to accentuate the benefits of eating the fish. More on this latter. The take home message here is that the bigger the fish the greater to amount of mercury you get per gram of the fish.
Mercury can also creep into Non-Seafood Foods:
Though less common, mercury can also be found in certain food crops due to uptake from contaminated soils or water.
Rice: Rice can absorb mercury from polluted soil and water. In areas where mercury is used or naturally occurring in high amounts, such as near gold mining sites or industrial areas, rice might absorb higher levels of mercury.
Vegetables and Fruits: Like rice, vegetables and fruits can absorb mercury from the soil, particularly in areas with high atmospheric mercury deposits that settle in the soil. Leafy vegetables tend to accumulate more heavy metals. Organic mercury compounds used historically in agriculture could also contribute to residual soil contamination affecting crops.
Meat and Poultry: Animals raised in contaminated environments can accumulate mercury. This is generally less significant compared to fish but can be a factor in areas with high environmental contamination.
High Fructose Corn Syrup (HFCS): Some studies have detected mercury in HFCS, attributed to caustic soda and hydrochloric acid used in its production, which can be contaminated with mercury. While HFCS is less common in Australian diets compared to the US, it's used in some imported processed foods.
Analysis of mercury in hair specimens can supply useful information about exposure to organic mercury compounds such as methyl mercury, particularly to help in reconstructing patterns of prior or seasonal exposure from fish consumption. However, it is important to consider all possible exposures to any forms of mercury when hair mercury levels are elevated.
Cosmetics:
We recently published the article below and highlighted that Mercury is indeed in some Cosmetics.
Cosmetics, Personal Care and Hygiene products in Australia - Heavy Metals and other contaminants
This is the first of many articles to come under the umbrella of Raising Awareness. We decided to publish this first because our next article, entitled - Excuse me waiter, there is Mercury in my fish, will refer to an element in this one. Yes, you guessed it - Mercury (Hg). After that the focus shifts to
Mercury detected in eye-liner
Only a few days ago (May 2024) a client sent in a sample of eye-liner powder to our Laboratory for analysis. Once the results are published we will show them here - initially though we detected Mercury (0.2 ppm) and Lead (0.4 ppm) in the sample.
Dentistry and Mercury Exposure
Mercury-based amalgams used for dental fillings can release small amounts of mercury vapour, which are inhaled and absorbed by the lungs.
FUN FACT
Nearly 20 years ago I had 11 mercury amalgams fillings removed over a period of several months from a very thorough dentist who specialised in this procedure and used the outmost precaution. Upon first arriving in his clinic we were sent to a room and asked to blow into a machine that was capable of detecting mercury vapour in your breath. I did as told and the reading dial on the machine hardly registered. I was very happy about this. Then I was given some chewing gum and asked to chew for a few minutes, followed by blowing again into the machine. This time the dial on the machine pegged to the end of the scale.
Mercury is released from older or deteriorating dental amalgams when we chew (mine were old).
When people chew or grind their teeth while sleeping, dental amalgams emit Mercury vapour, which upon inhalation is absorbed and moved into the blood stream.
The World Health Organization (WHO) has recommended phasing out dental amalgams (the silvery filling) and change to alternative materials. Occupational dental personnel who work with amalgams are at risk of exposure to undesired metallic Mercury levels.
Mercury exposure for dentists has been associated with reproductive disturbances and allergies and with neurological and/or cognitive problems, fibromyalgia, and chronic fatigue syndrome.
Norway and Sweden have banned the use of amalgam. Japan and other EU countries are considering ban on amalgams. In France, Finland, Austria, Germany, and Canada, the use of dental amalgam have purposely been reduced for children, pregnant women, and subjects with kidney problems.
Several studies have shown that dental personnel have higher Hg concentrations in urine than the average population and that dental nurses had higher Hg values than dentists. After occupational exposure to Hg vapour even at low doses, inorganic Mercury is found in the brain of dental personnel with autopsys’ undertaken later revealing significant inorganic Mercury deposits remaining in the brain.
Mercury exposure and health impacts in dental personnel
Industrial Exposure:
Coal-fired Power Plants: See below for lots of detail.
Mining and Smelting Operations: These activities release mercury directly into the environment.
Waste Incineration: Burning waste can release mercury that was present in discarded products.
Consumer Products:
Various products including batteries, lighting (e.g., fluorescent bulbs), and electronics may contain mercury. Used in fluorescent lighting because electricity passed through mercury vapour in a fluorescent lamp produces short-wave ultraviolet light, which then causes the phosphor in the tube to fluoresce, making visible light.
Disposal and breakdown of these products can release mercury into the environment.
Residential Exposure: Use of certain traditional medicines and presence of mercury in some paints and skin creams including skin whitening creams.
Pharmaceuticals
Mercury use in Pharmaceuticals is a contentious issue.
To date, there is no consensus on Thimerosal safety in Vaccines.
Thimerosal, an organo-mercury compound, containing ethyl mercury, has been used as a preservative in vaccines and other pharmaceutical products since its development in 1927. It has been used to prevent bacterial and fungal growth in vaccines. Concerns about its potential neurotoxic effects, especially in children, have led to scrutiny over the years.
Due to public concern, thimerosal has been removed or reduced to trace amounts in all childhood vaccines in the United States, except for some formulations of the flu vaccine. In many other countries, similar measures have been taken to reduce or eliminate thimerosal from vaccines administered to children.
Despite concerns and reductions in its use, thimerosal continues to be found in certain vaccines (especially some flu vaccines), as well as in other products like nasal sprays, eye solutions, and antiseptic ointments.
It's important to note that the use of thimerosal has decreased significantly in many countries, particularly in vaccines for children, due to ongoing safety reviews and public concern about potential health risks, including neurotoxicity.
Despite the recognised neurotoxicity of thimerosal, it remains in use in these products primarily because of its perceived effectiveness as a preservative.
In both Australia and the United States, thimerosal is primarily used in some multi-dose vials of flu vaccines. The reason for its use in multi-dose vials is to prevent contamination when the vial is punctured multiple times.
In Australia, one of the flu vaccines that may contain thimerosal in its multi-dose vial form is Fluzone. This is similar to its use in the United States.
The Fluzone Quadrivalent multi-dose vial contains thimerosal as a preservative. Each 0.5 mL dose from the multi-dose vial includes 25 micrograms of mercury. Other ingredients include hemagglutinin from four different influenza strains, formaldehyde, and egg protein. The multi-dose vial is designed to prevent contamination when used multiple times (Drugs.com).
Fluzone is manufactured by Sanofi Pasteur, a major global vaccine producer.
Thimerosal is a mercury-containing organic compound used as a preservative in some vaccines. It is composed of ethylmercury and thiosalicylate. The ethylmercury in thimerosal is the form of mercury that acts as an antimicrobial agent. Eli Lilly and Company originally developed thimerosal, but various chemical suppliers now produce it.
Thimerosal is used in various pharmaceutical products beyond vaccines. It is often found in:
Nasal Sprays: Thimerosal serves as a preservative in some nasal sprays to prevent microbial contamination.
Eye Solutions: It is used in certain ophthalmic solutions to extend shelf life and reduce the risk of bacterial and fungal growth.
Antiseptic Ointments: Thimerosal is included in some topical antiseptic solutions and ointments for treating cuts and wounds due to its antimicrobial properties.
Thimerosal (Merthiolate) is a well-known preservative used in pharmaceutical products, the safety of which was a matter of controversy for decades. Thimerosal is a mercury compound, and there is a debate as to whether Thimerosal exposure from vaccination can contribute to the incidence of mercury-driven disorders. To date, there is no consensus on Thimerosal safety in Vaccines.
According to our results Thimerosal should be present in culture media at 100 μg/ml (250 µM) concentration to achieve an effective antimicrobial activity. We showed that this amount of Thimerosal is toxic for human and animal cells in vitro since the viability of all examined cell lines was suppressed in the presence of less than 5 μg/ml (12.5 μM) of Thimerosal. Overall, our study revealed Thimerosal was 333-fold more cytotoxic to human and animal cells as compared to bacterial and fungal cells. Our results promote more study on Thimerosal toxicity and its antimicrobial effectiveness to obtain more safe concentrations in biopharmaceuticals.
From - In vitro assessment of Thimerosal cytotoxicity and antimicrobial activity 2023
Also see -
A very interesting article that gives a detailed historical account of the development, use and evidence of Thimerosal efficacy - A Review of Thimerosal (Merthiolate) and its Ethylmercury Breakdown Product: Specific Historical Considerations Regarding Safety and Effectiveness 2007
Examining the evidence that ethylmercury crosses the blood-brain barrier, 2020
Assessment of thimerosal effects in sublethal concentrations on zebrafish (Danio rerio) embryos exploring NMR-based metabolomics profile, 2024
Medical & Laboratory chemicals and equipment
Mercury is used in thermometers, barometers, manometers, sphygmomanometers, float valves, mercury switches, mercury relays, though mercury in thermometers and sphygmomanometers is being phased out.
Mercury in Laboratories
Chemical Reagents: Mercury is a component of various laboratory reagents used in chemical analysis and research. These include reagents for high-performance liquid chromatography (HPLC) and other analytical techniques.
Equipment: Some laboratory devices and instruments, such as barometers, manometers, and some switches, contain mercury. The risk of exposure arises if these instruments break, causing mercury spills.
Research Use: Mercury and its compounds are used in research for the study of their effects on biological systems, in synthesis of chemical compounds, and sometimes in preservatives and fixatives.
Natural Sources:
Volcanic eruptions and forest fires can release mercury naturally present in the Earth into the atmosphere.
Artisanal and Small-Scale Gold Mining (ASGM):
This process often involves the direct handling of mercury to extract gold from ore, leading to significant direct exposure. Plus its contamination of regional rivers that then flow into the ocean - as mentioned previously by the waiter.
Occupational Exposure:
Jobs involving mercury handling or exposure to mercury-containing materials (like in dental, health, or industrial settings) pose risks.
Atmospheric Deposition: Burning coal deposits Mercury (Hg) into our oceans
Mercury, in addition to other chemicals of concern, are emitted into the atmosphere and deposits into oceans, rivers, lakes, and land , leading to bioaccumulation of mercury in the food chain.
With the growing concern about climate extremes and coal burning, little attention is paid to the continual contribution of burning coal to atmospheric deposits of chemicals into our ocean — particularly Mercury.
Most of the mercury found in the oceans comes from several sources, both natural and human-made:
Atmospheric Deposition: This is the largest source of mercury in the oceans. Mercury released into the atmosphere through various processes eventually settles into the oceans. This atmospheric mercury primarily originates from human activities such as burning coal, mining, and industrial processes. Natural sources include volcanic eruptions and the degassing of the Earth's crust.
Hydrothermal Vents: Deep-sea hydrothermal vents release mercury directly from the Earth's crust into the ocean water.
Overall, human activities have significantly increased the amount of mercury in marine environments compared to natural levels. Atmospheric deposition remains the predominant pathway through which mercury enters marine ecosystems. Oh, that’s what the waiter said!
The proportion of atmospheric deposition from burning coal and the sort of coal releasing the most mercury
Atmospheric deposition of mercury is significantly influenced by the burning of coal, and this activity is one of the largest anthropogenic (human-made) sources of mercury emissions globally. The exact proportion of mercury in atmospheric deposition that comes from coal burning can vary by region and depends on local industrial activities and regulations. Generally, it is estimated that coal combustion for power and heat is responsible for about 40% to 50% of total global anthropogenic mercury emissions.
Regarding the type of coal, the mercury content can vary significantly:
Lignite (Brown Coal): Typically has a lower heat content and often a higher moisture content. It contains relatively more mercury compared to other types of coal and releases more mercury per unit of energy generated.
Bituminous Coal: This type of coal has a higher heat content and less moisture compared to lignite. It often has a moderate amount of mercury but because it is more widely used, especially in power plants, it contributes significantly to mercury emissions.
Sub-bituminous Coal and Anthracite: These have lower mercury contents compared to lignite and bituminous coal. Anthracite has the highest heat content and lowest moisture and typically contains the least mercury.
The release of mercury from coal also depends on the combustion technology used, the presence of mercury removal technologies at the combustion site, and the specific geological origin of the coal. Coal-fired power plants in regions without stringent environmental controls tend to release more mercury into the atmosphere.
The main type of coal used and burned in Australia
Australia primarily uses and exports bituminous and sub-bituminous types of coal. Bituminous coal is widely used due to its high heat content and efficiency in electricity generation. Sub-bituminous coal, which has slightly lower energy content compared to bituminous, is also significant in the energy mix.
Australia is one of the world’s largest exporters of coal, and its coal resources are predominantly of these two types. Additionally, Australia also has deposits of lignite, also known as brown coal, which is mostly used domestically, particularly in power stations in the state of Victoria. However, bituminous and sub-bituminous coals are the main types when considering both domestic use and export.
Environmental and Accidental Releases:
Spills and improper disposal of mercury and mercury-containing items can lead to localized environmental contamination.
Specific Considerations for Australia:
Industrial and Mining Activities: Australia's extensive mining industry, including gold and coal, could contribute to localized mercury emissions. This can affect local ecosystems and potentially lead to higher mercury levels in locally sourced produce and grains.
Environmental Regulations: Australia has strict regulations regarding emissions and contaminants, including mercury. The Australian government also participates in international agreements like the Minamata Convention to reduce mercury emissions.
Fish and Seafood: Australia provides guidelines on the safe consumption of fish, especially for pregnant women and young children, due to mercury concerns. These guidelines are crucial given the popularity of fish like shark (flake) and swordfish, which are known to have higher mercury levels. Food Standards Australia New Zealand (FSANZ) monitors chemical residues in food and provides guidelines to minimize risks associated with mercury and other contaminants. More on this in PART 3.
Some guidelines from the USA
The safe upper limit of mercury consumption is about 0.1 microgram per kg of body weight per day, according to the U.S. Environmental Protection Agency (EPA). The U.S. Food and Drug Administration think the safe dose is higher than that, more like 0.3 micrograms/kilogram/day. So if you weigh 68 kilograms, that's somewhere between 6.8 and 20 micrograms per day, or 48 to 140 micrograms per week.
NOTE: at this point we still think this is too high! More on this in PART 3 of this series.
More about our Main Character
Three major forms of mercury
Mercury is a heavy metal found in different forms, each with distinct properties, toxicity levels, and sources of exposure. Here’s a detailed breakdown of the three major forms of mercury - Methylmercury, Inorganic Mercury Compounds, and Elemental Mercury + sources of exposure.
Hg is a liquid at room temperature, known as metallic or elemental Mercury and is used in dental amalgams and various domestic and industrial applications.
Unfortunately, at room temperature, metallic or elemental Mercury gives off a gaseous form of Hg that is lipophilic (attracted to fatty tissue) and readily absorbed upon inhalation and then easily able to cross the placenta or the blood-brain barriers.
To make matters worse, once Mercury is in a human cell, it is rapidly oxidized by a catalase enzyme into inorganic Mercury (Hg2+), which in the neuron cells of the brain can be retained for years. Organic Mercury compounds like Methylmercury (MeHg) from fish or Ethyl mercury from the compound Thimerosal found in some vaccines (especially flu-vaccines) and Phenyl mercury can, however, penetrate into the blood brain barrier whereupon they are converted to inorganic Hg and then trapped in the brain or nervous tissue for years.
Adding fuel to this fire of body mercury bio-accumulation, is the realisation that individuals can be exposed to multiple sources of mercury at once.
1. Methylmercury
Methylmercury is an organic compound of mercury. It is the most toxic form of mercury and is known for its ability to bioaccumulate in fish and aquatic life. It is formed when inorganic mercury is methylated by bacteria in aquatic systems.
Sources of Exposure:
Dietary Intake: The primary source of exposure for humans is through the consumption of fish and shellfish contaminated with methylmercury. Predatory fish higher up in the food chain (such as shark, swordfish, king mackerel, and certain species of tuna) tend to have higher concentrations.
Occupational Exposure: Although less common, people working in industries dealing with mercury can also be exposed to methylmercury, though most occupational exposure tends to involve other forms of mercury.
2. Inorganic Mercury Compounds
Inorganic mercury compounds can be either mercuric chloride or mercuric oxide, among others. They are generally less volatile than elemental mercury but can still be highly toxic, particularly if ingested or absorbed through the skin.
Sources of Exposure:
Skin Contact: Exposure to inorganic mercury compounds can occur through the use of some skin creams and antiseptics that contain mercury.
Dietary Intake: While less significant than methylmercury, inorganic mercury compounds can also be found in food, though this is generally due to contamination during processing or from the environment.
Occupational and Environmental Exposure: Workers in industries such as chloralkali plants, mining, and others where mercury is used or produced may encounter these compounds. Inorganic mercury can also be released into the environment from industrial processes and contaminate air, water, and soil.
3. Elemental Mercury (Metallic Mercury)
Elemental mercury is the pure, metallic form of mercury, also known as quicksilver. At room temperature, it is a shiny, silver-white, odorless liquid that can evaporate to become an invisible, odorless, toxic vapor.
Sources of Exposure:
Dental Amalgams: Dental fillings containing mercury can release mercury vapor during chewing or when they are placed or removed.
Thermometers and Barometers: Older thermometers, barometers, and other devices that contain elemental mercury can be sources of exposure if they break and release mercury vapor.Occupational Exposure: Industries that use or produce elemental mercury, such as gold mining (particularly artisanal small-scale gold mining) and manufacturing, pose risks to workers through inhalation of mercury vapors.
Cultural and Ritualistic Uses: In some cultures, elemental mercury is used for ritualistic purposes, which can lead to direct exposure.
In PART 2 we cover the Health Effects of Mercury(Hg) in Humans
Please leave comments, ask questions and share this article.
Thank you. Hartmut Gunther, May 2024